Measuring hydrogen sulfide concentration in a pH-volatile liquid (such as water) is much more difficult than in a medium like natural gas. As the pH increases, the H2S dissociates into its ions HS- (bisulfide) and S2- (sulfide), which are not measured by a typical H2S sensor. A reading that doesn’t account for the presence of these other ionic forms is meaningless at high pH because it would grossly understate the total H2S loading of the fluid when the pH drops.
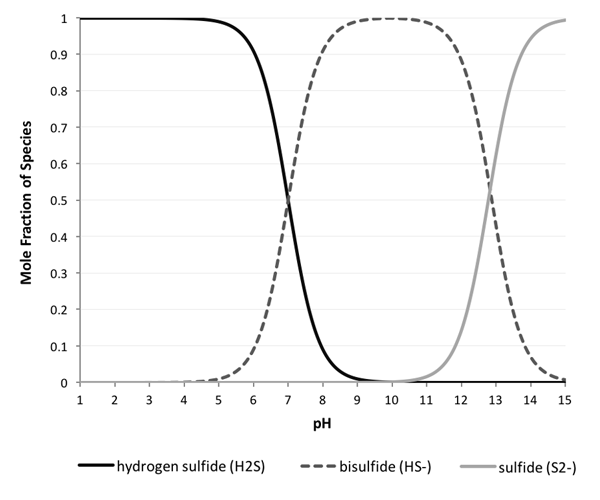
H2S and pH: As visualized above, H2S begins to dissociate at a pH of ~5, and forms 100% HS- at a pH of ~9. At a pH above 15, we see 100% S2-.
There are a few common tricks for compensating for the dissociation to get an accurate online reading. You can add a strong acid (typically HCl or H2SO4) to the sample loop and force the pH below the dissociation point, but the problem there is obvious: the consumable acid is extremely expensive over time.
Others have tried using a pH meter along with an H2S sensor to predict the concentration of HS- based on an ionic fraction curve and the current pH reading; i.e., if you measure the H2S at 50 ppm and the pH at 7, you could predict 50 ppm of HS-. People who have tried this method have learned the hard way that H2S poisons the pH sensor’s electrodes by corrosion and precipitation with the silver in the reference element. Even with the most resilient electrodes money can buy, the pH reading is not a solid basis for correlation: non-H2S species in the stream can alter the acidity, and pH can be far from homogenous in a fluid with high flow rate.
pH-Independent Online Measurement of H2S in Liquids
What if you ignored the pH of the sample completely and simply measured H2S, HS-, and S2-, independently and simultaneously? This kind of measurement used to be confined to the domain of the lab. Applied Analytics' multi-component technology brings this measurement to online process control.
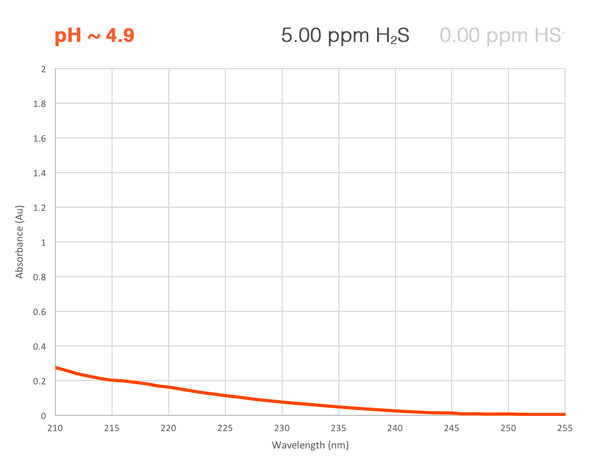
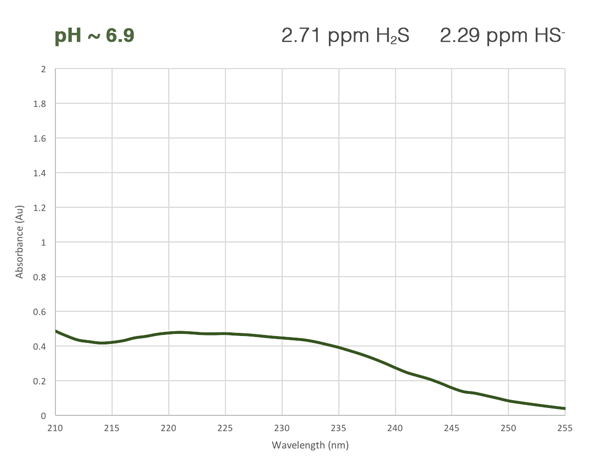
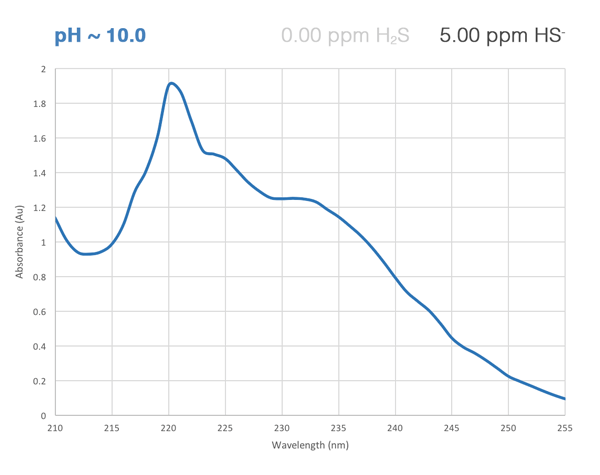
Each of the ionic forms of H2S has its own unique absorbance curve in the UV wavelength region. Using a high-res UV-Vis spectrophotometer and a multi-component calibration, the OMA H2S analyzer measures the total sample absorbance and mathematically isolates the absorbance of each species. This direct measurement gives an accurate reading of total hydrogen sulfide + bisulfide + sulfide in the fluid, regardless of the current equilibrium. Best of all, we achieve a relentlessly reliable reading without depending on expensive consumables or short life pH sensors.
Specific Use Cases
The reason for measuring H2S and its dissociated forms is, of course, to manage how you remove it from your process stream. Depending on the amount of H2S saturation, the fluid composition, the geographic location, and your budget, an H2S removal operation can range from adding a bit of activated carbon to using a full-blown sulfur recovery unit.
The pH-independent multi-component method pioneered by Applied Analytics shines in very specific use cases. While you will never need to measure all three ionic forms at once (there is no pH at which all three exist together), you may have a process stream where the pH varies so greatly that you encounter all three ionic forms at different times. The versatility and accuracy of the 3-component calibration makes the OMAhighly suitable for heterogeneous streams.
Groundwater Groundwater treatment plants need to measure H2S both in the water released (for compliance) and water received (for treatment optimization). The source of this 0-10 ppm H2S contamination is rock and sediment in the surrounding environment. Effective H2S management has traditionally required constant pH monitoring, usually around a set point of 7, where H2S and HS- exist together; using a pH-independent OMA H2S Analyzer, no knowledge of the pH is required since the measurement automatically totals H2S and HS- concentration.
Caustic Desulfurization
In the petrochemical industry, caustic (i.e. NaOH) is a tried-and-true method for removing high levels of H2S from liquid streams. By adding the strong base, you keep the pH of the stream above a set point where 100% of the H2S exists as S2-. This way, you can ensure that none of the sulfur escapes the fluid by evaporation, and that 100% of the dissociated H2S can be effectively precipitated into a removable salt.
In this type of operation, you can either dump expensive caustic blindly or you can use analytics to optimize and save money. At such high H2S saturation, pH sensors can become unstable within a single day. The pH-independent OMA H2S Analyzer uses a multi-component analysis to directly detect the failure condition which a pH meter merely predicts: the formation of HS- in the stream. Using Applied Analytics technology, you can optimize caustic use in real time by monitoring the HS- absorbance curve and ignoring pH altogether